Calculations Involving Colligative Properties Freezing Point Depression and Boiling Point Elevation Calculations. - ppt download

Calculate the biling point of a solution containing 0.456 g of comphor (molar mass=152 g mol^(-1)) dossoved in 31.4 g of acetone(b.p.= 329.45 K). Given that the molecular elevation constant per 100
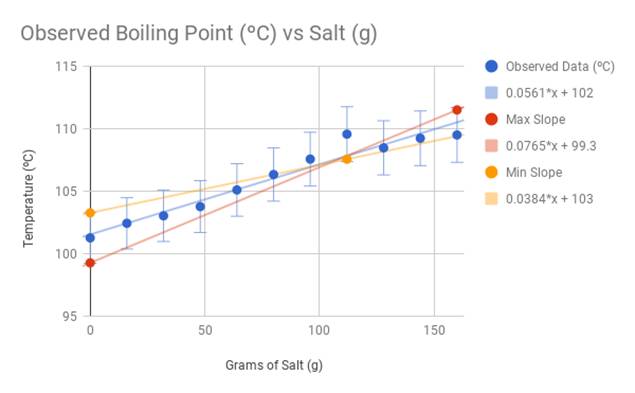
Calculate the molal elevation boiling point constant of a solution containing 1.0 gm of urea (M = 60) in 75.0 gm water and boils at - Sarthaks eConnect | Largest Online Education Community
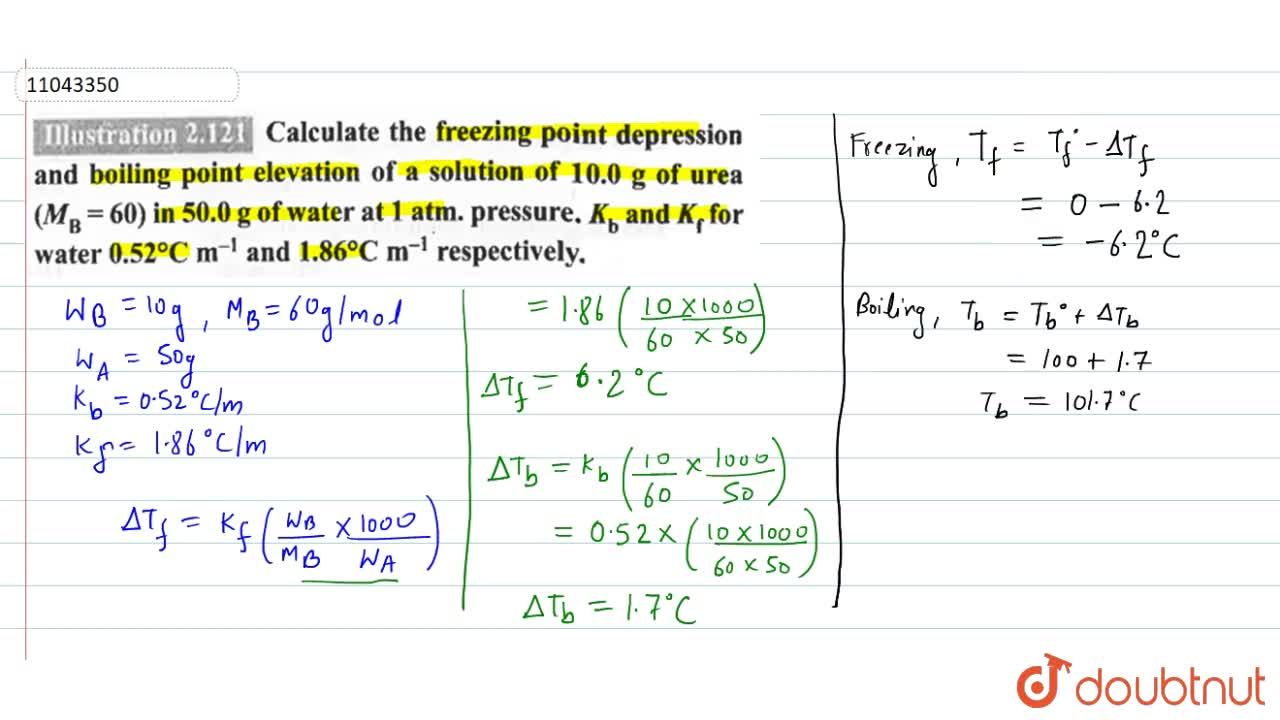
Calculate the freezing point depression and boiling point elevation of a solution of 10.0 g of urea (M(B)=60) in 50.0 g of water at 1 atm. pressure.K(b) and K(f) for water 0.52^(@)C

SOLVED: The elevation in boiling point, when 0.30 g of acetic acid is dissolved in 100 g of benzene is 0.0633OC. Calculate the molecular weight of acetic acid from this data. What
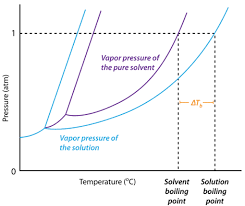
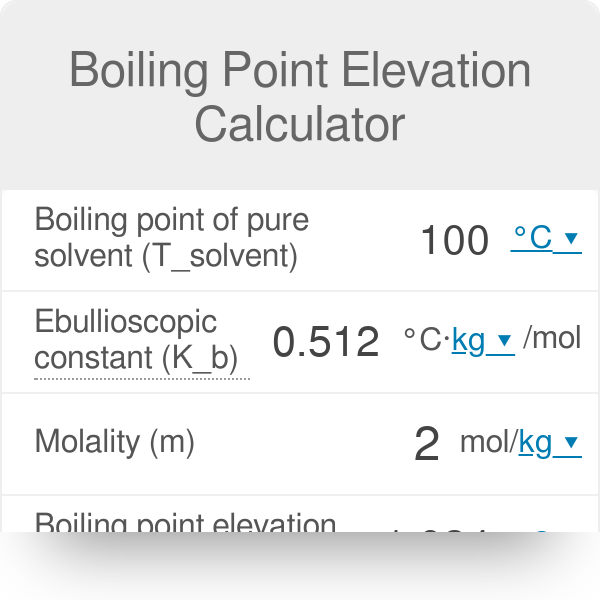
How to Calculate and Solve for Van't Hoff Factor, Ebullioscopic Constant, Molality and Boiling Point Elevation | The Calculator Encyclopedia - Nickzom Blog

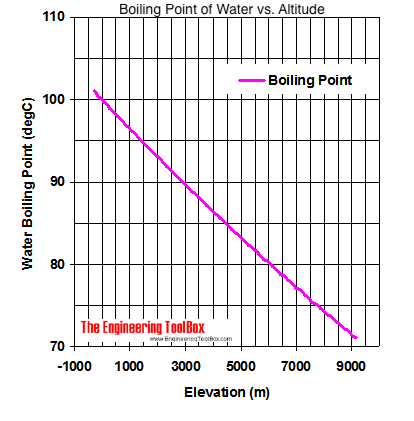
Calculate the freezing point and the boiling point at 1 atmosphere of a solution containing 30 g cane sugar (molecular mass 342 ) and 150 g water.Given : Kb = 0.513 and Kf = 1.86